
MARCH 18, 2019
New study from a team of scientists led by Dr. Lin Wang of HPSTAR investigated the stoichiometry and structural evolutions of phosphine (PH3) under high pressures. They found that PH3 molecules went through a step-by-step polymerization and finally dissociated to elemental phosphorus and hydrogen instead of directly dissociation as previously reported. Low temperature was found to greatly hinder the polymerization/decomposition of phosphine. The results also revealed that the decomposed product P4H6 might be responsible for the reported superconducting transition. The study is published in recent National Science Review.
Most known superconductors — a material through which electricity can flow with zero resistance, only function at super-low temperatures which greatly hinder the practical applications. Looking for high–temperature superconductor is scientists’ long-standing aim. Phosphine—PH3, is a superconductor found in 2015, a potential high-temperature superconducting candidate.
However, the structure and stoichiometry are found to be very complicated and the origin of the superconducting phase still remains puzzling. “It’s very challenging to characterize the structural information at super-high pressures simultaneously with transportation measurement”, said Dr. Lin Wang.
Previous studies show that phosphine will break down under compression like what does in H2S. So phosphine's superconductivity under compression was proposed to arise likely due to the decomposed products instead of phosphine itself. Then the stoichiometry and structure of the decomposed products are the key questions for compressed phosphine.
In a recent research from an international team of scientists led by Dr. Lin Wang from HPSTAR set out to probe the possible stoichiometry and structure evolutions of phosphine up to above 2 million of atmospheric pressures by using in-situ spectroscopy measurements with theoretical calculations.
They found that phosphine did not decompose directly while it went through two polymerizations and then decomposed. First phosphine polymerized into P2H4 and then P2H4 further polymerized into P4H6. With further compression at 35 GPa, P4H6 decomposed into elemental phosphorus and hydrogen.
It’s interesting that low temperature can greatly hinder phosphine’s polymerization/decomposition under high pressure. P4H6 could be stable up to at least ~205 GPa where the highest superconducting temperature reached. So they proposed that P4H6 might be responsible for superconductivity at high pressures.
Their further calculations also support that P4H6 might be the corresponding superconductor phase—the superconducting temperature for P4H6 agrees well with the reported results.
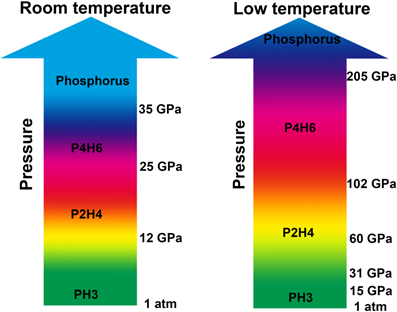
Caption: The high pressure phase diagrams of PH3 at room temperature and low temperature.
磷烷(PH3)也是一种典型的氢化物,在高压下PH3可以转变为超导体。然而PH3在高压下的结构及对应的超导相尚不清楚。最近,北京高压科学研究中心王霖研究员带领的国际研究小组结合原位高压实验研究和第一性原理计算的方法,对PH3在高压下的结构和化学计量比的演化进行了细致研究。他们对PH3在高压下的结构相变进行了表征,发现室温下PH3只可以稳定保持到11.7 GPa,之后发生聚合反应生成P2H4。在25 GPa,P2H4会再次聚合生成P4H6。当压力高于35 GPa,P4H6会分解为磷单质和氢气。另外他们发现低温能有效抑制磷氢在高压下的聚合和分解。低温下,P4H6能稳定保留到205 GPa。他们进一步的计算表明P4H6在高压下可以转变为超导体,并且与实验上发现的PH3超导转变温度相符。相关研究结果发表于《国家科学评论》(National Science Review)。