
MAY 21, 2019
An international team of scientists including Dr. Xujie Lü has developed a new halogen conversion–intercalation chemistry in graphite that produces composite electrodes with a capacity of 243 mAh g-1 at an average potential of 4.2 volts versus Li/Li+. The study is recently published in Nature.
State-of-the-art Li-ion batteries are facing safety and stability concerns due to the utilization of organic electrolytes. The use of ‘water-in-salt’ electrolytes has considerably expanded the electrochemical window of aqueous lithium-ion batteries to 3 to 4 volts, making it possible to couple high-voltage cathodes with low-potential graphite anodes. However, the common transition-metal-oxide cathodes have very limited lithium intercalation capacities which preclude the improvement of energy densities. Some anionic redox reactions may help achieve higher capacity, but at the expense of reversibility.
The team of scientists designed a reversible halogens intercalation in graphite structures using the anionic-redox reaction from a concentrated aqueous electrolyte. They mixed anhydrous LIBr and LiCl with graphite at a 2:1:2 molar ration and prepared a composite cathode material, (LiBr)0.5(LiCl)0.5-graphite. The highly concentrated water-in-bisalt electrolyte partially confined hydrated LiBr/LiCl within the solid cathode matrix, and upon oxidation, Br0 and Cl0 are stabilized by sequential intercalation into the graphite host as solid graphite intercalation compounds.
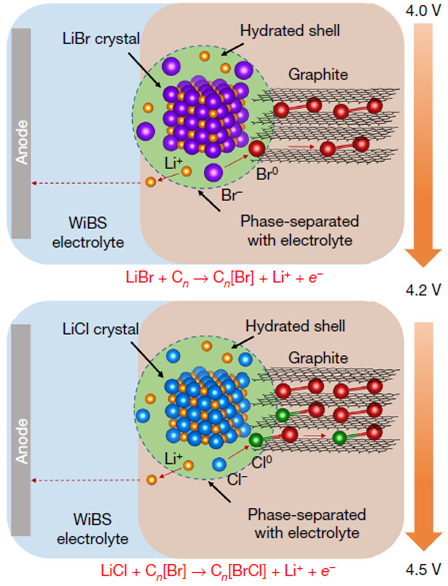
Their experimental characterization and theoretical modeling revealed that the high capacity/energy density is due to the formation of the graphite intercalation compound which can form reversibly in water-in-salt electrolyte. This new cathode chemistry inherits the high energy of the conversion reaction and the excellent reversibility of topologic intercalation.
Combining this cathode with a graphite anode, the team created a aqueous Li-ion full battery with a high potential of 4V and a high energy density of 460 Wh kg-1, as well as a nearly 100% Coulombic efficiency. Such an anion conversion-intercalation mechanism integrates the merits of high energy densities of the conversion reactions, the excellent reversibility of the intercalation mechanism, and the improved safety of aqueous batteries.
Caption: Proposed conversion–intercalation chemistry.
越来越多研究人员开始将盐包水(water-in-salt)电解质用于锂离子电池来扩展电化学窗口到3至4V,从而使得高压正极与低电位石墨负极可以配对使用。然而,典型的过渡金属氧化物正极中,锂嵌入容量小,限制了能量密度的进一步提高。部分阴离子氧化还原反应可以实现更高的容量,但是不具有可逆性。最近,北京高压科学研究中心吕旭杰研究员参与的新研究提出了一种全新的卤素插层水系电池。该研究创造性的利用卤素阴离子(Br-和Cl-)在石墨中的氧化还原反应,将无水LiBr和LiCl以及石墨在2:1:2 的最佳质量比条件下混合,制备得到一种含有等摩尔卤化锂盐(LiBr)0.5(LiCl)0.5—石墨的全新复合电极(LBC-G)。这种全新的正极化学方法兼具转化反应的高能量和拓扑嵌入的优异可逆性,称为转化-插层化学机制。这种阴离子转换-插层机理不但可以提高锂离子电池的能量密度,同时具备优异的循环性能和良好的电池安全性。