
JULY 2, 2019
Sodium-sulfur (Na-S) batteries are promising candidate for solid state batteries due to their high ionic conductivity, long cycle-life, high specific energy, and low material cost. A systematic and unambiguous understanding of the charging/discharging process in batteries is of great significance for the optimization of Na-S batteries. However, the fundamental structure understanding of sulfur-rich Na-S phases in charging/discharging process remains elusive.
A team of scientists co-led by Dr. Huiyang Gou of HPSTAR performed a systematic structure searches and electronic properties calculations at ambient and high pressure up to 50 GPa to Na-S system using first-principles computations and extensive structural searches. Except for the known compounds, they found four new compounds —Na3S, Na5S3, Na2S2, and Na2S3.
Among them, the previously unidentified Na2S3 shows thermodynamically metastability under ambient pressure, while with much lower formation energy relative to Na2S2 and Na2S4 at 0 GPa and can be stable above 0.9 GPa. “This will allow Na2S3 to exist under non-equilibrium conditions, such as electrochemical process,” explained by Biao Wan, one of the co-first authors.
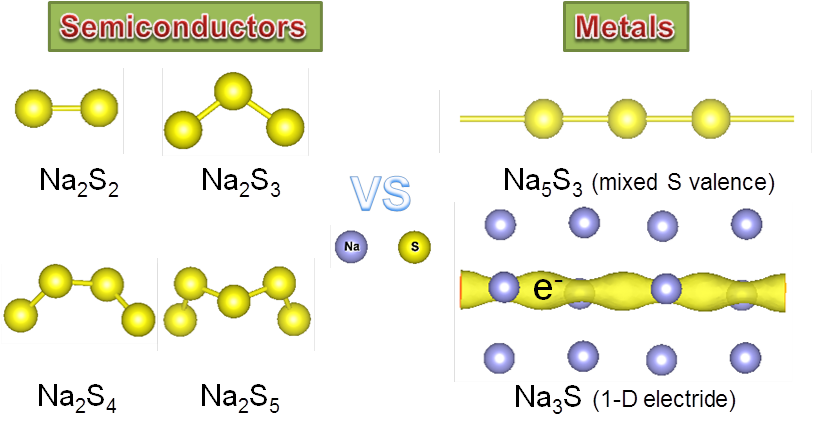
Interestingly, Na3S and Na5S3 show intrinsic metallic characters, distinctly differing from the other Na-S semiconducting phases. “The two phases have totally different electrical pathways—a one-dimensional electron gas in the channel voids in Na3S and an infinite sulfur chains with metallic S-S bonding in Na5S3,” explained Dr. Huiyang Gou.
The one dimensional electron gas makes Na3S to be a potential one-dimensional electride. “Electride is an interesting class of ionic solids with electrons as anions,” Dr. Huiyang Gou explained. “Na3S that we discovered in this work is the first Na-S electride.”
Moreover, they found that sulfur in Na5S3 exhibits a mixed valence state with -2 and -1 — the first mixed valence states that found in alkali metal sulfides.
The team also simulated the voltage that go through Na2S4 to generate Na2S3 in a battery, which is consistent with the experimental measurement.
Their study may help to discover other new candidates in Na-S system and elucidate the potential electrochemical mechanism in the Na-S battery. The work was published in recent of Journal of Materials Chemistry A.
“Our study suggests that high pressure is an effective approach to identify metastable compounds and could help to explore novel electrode materials with excellent electron conductivity.” added by Dr. Huiyang Gou.
Caption: Diverse structures of Na-S system
Li-S及Na-S电池具有高容量,高离子导电性,以及长循环寿命而备受人们关注。由于相对成本较低,Na-S电池在最近几年引起了科研人员的广泛兴趣,并在工业生产中得到了应用。研究在电化学循环过程中产生的Na-S化合物对深入了解Na-S电池工作原理具有重要意义。作者通过第一性原理结合结构搜索方法,对Na-S体系在常压和高压下的相稳定性及电子结构进行了系统性的研究。发现了四种新的Na-S亚稳结构: Na3S, Na5S3, Na2S2和Na2S3。其中Na2S3的发现为寻找电化学过程中的中间产物提供了思路。另外他们发现Na3S, Na5S3表现出金属的性质。这是首次在Na-S半导体体系中发现金属化合物。进一步的计算表明Na5S3与一维电子化合物Na3S表现出两种完全不同的导电机理:Na5S3主要靠金属键连接的无限长S-S链,而Na3S则依赖局域在Na原子形成的通道里的间隙一维电子气来导电。这些化合物的发现,对于设计和发现新型Na电池电极材料具有重要意义。