
JUNE 23, 2020
The glass structure of a material is often believed to mimic its corresponding liquid. Polyamorphism between ices has been used as a guide to elucidate the properties of liquid water. But how many forms of amorphous ices are there? Do we understand how metastable high-pressure crystalline ice evolves towards the thermally stable low-density form? An international research team led by Chuanlong Lin and Wenge Yang from HPSTAR has revealed a multiple-step transformation mechanism using state-of-the-art time-resolved in situ synchrotron x-ray diffraction. A temperature/time-dependent kinetic pathway with three distinctive transitions was identified in the structural evolution from metastable crystalline ice (ice VII or ice VIII) to the thermodynamically stable ice I. These intermediate processes compete against each other. The end result is a juxtaposition of these processes. The work is published in PNAS.
Water plays a vital role in the origin of life on Earth. In the liquid phase, it exhibits many unusual properties. In the solid phase, ordinary ice also displays diverse phase transitions at high pressure. Many theoretical and experimental studies have been devoted to understanding the underlying inter-conversion mechanisms. So far, most experiments have been ex situ measurements on recovered samples and lack detailed information on the structural evolution accompanying the transformation. Previous studies have been hindered by technical difficulties in monitoring the rapid structural change over a broad pressure and temperature range.
In 2017, Lin and colleagues overcame the experimental challenge. A series of studies was conducted to investigate ice transitions by combining in situ time-resolved x-ray diffraction, and remote pressure control with different ramp rates within a low-temperature cryostat. This capability allowed the suppression of thermally-driven crystalline-crystalline transitions [PNAS 115, 2010-2015(2018)]. Important insights into the complexity of the poly-amorphous transformations were obtained, such as the kinetically-controlled two-step amorphization in ice Ih [Phys. Rev. Lett. 119, 135701(2017)] and the successful venture into the no man’s land [Phys. Rev. Lett. 121, 225703(2018)].
Caption: An illustration shows structural evolution of ice VII as a function of time at constant P-T conditions.
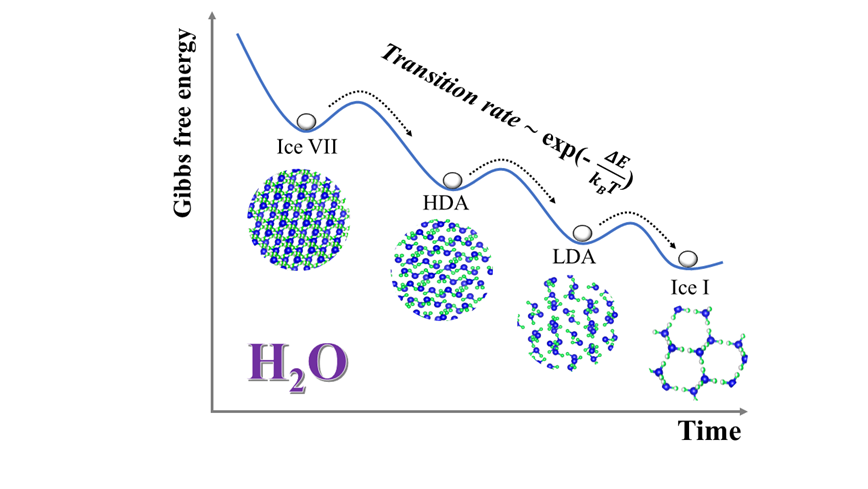
Now, we try to answer what exactly is the nature of the amorphous-amorphous phase transformation processes? Using the newly developed techniques, we explored the 'mirror' process, i.e., reverse transformation from a meta-stable high-density crystalline ice (i.e, ice VII or ice VIII) to the ambient stable ice I. We identified the temperature/time-dependent kinetic pathways and characterized the interplay/competition between the high density amorphous (HDA)-low density amorphous (LDA) transition and recrystallization. Contrary to previously reported ice VII (or ice VIII) → LDA→ ice I transformation sequences, time-resolved measurements show a three-step process: initial transformation of ice VII to HDA, followed by a HDA-LDA transition, and then crystallization of LDA into ice I. Both the amorphization of ice VII and the HDA®LDA transition show distinctive thermal activation mechanisms. Significantly, both processes exhibit the Arrhenius behavior with a temperature-dependent duration time (τ) and a 'transition' temperature at around 110-115 K.
Large-scale molecular-dynamics calculations support the experimental findings. Furthermore, it shows the HDA ® LDA transformation is continuous with a large density difference and involves substantial displacements of water in the nano-scale. This study presents a new perspective on the metastability and complexities in shaping ice-transition kinetic pathways.
冰,作为水的固态形式广泛存在于我们的周围。但是看似普通的冰实际上是非常复杂的固体¾已知的就有17种不同的冰。那么不同的冰是如何互相转变的呢?近日,由北京高压科学研究中心林传龙、杨文革研究员和加拿大萨省大学John S. Tse教授主导的国际合作团队利用先进的时间分辨原位同步辐射X射线衍射技术揭示了冰的多步相变机制。他们在低压条件下通过观测冰从亚稳态晶体结构(ice VII或ice VIII)到热力学稳定态(ice I)的结构演化过程,发现了依赖于温度和时间且存在三种独特中间过程的动力学相变路径。这些中间过程相互作用、相互竞争,最终导致了并列共存。该成果以自由投稿形式发表于《美国国家科学院院刊》。