
Recently, a research team led by Drs. Ho-kwang Mao, Haiyan Zheng and Kuo Li from Center for High Pressure Science and Technology Advanced Research (HPSTAR) synthesized ultrafine diamond nanothreads with perfect carbon/nitrogen ordered structure through a distinctive reaction of aromatic systems under high pressure, and thus proposed a new method to synthesize more novel structure-specific carbon materials. This work was published on recent Proceedings of the National Academy of Sciences (DOI:10.1073/pnas.2201165119).
Carbon nanothreads (CNTh) is a special diamond-based material, in which the way of chemical bonding between carbon atoms is similar to diamond, so it has comparable hardness, insulation, stability and other excellent properties.
However, unlike the three-dimensional network structure of normal diamond, diamond nanothreads is one-directional, ~0.5 nanometers in diameter, ~one hundred thousand times smaller than a human hair (~50 microns). This special structure allows the diamond nanothread having equal to or higher tensile strength than that of carbon nanotubes, while also being extremely flexible.
The first CNTh was synthesized by polymerization of compressed benzene in 2015. Afterwards scientists synthesized series of CNTh containing various elements under high pressure, starting from various aromatic molecules. However, the obtained CNTh are usually mixtures with poor crystallization, which greatly hinders its practical application.
"The reason for the poor ordering of the nanothreads obtained under high pressure is because the selectivity of the reaction is not specific. Precise synthesis and the specific structure of the diamond nanothreads depends on the reaction selectivity of the raw materials, which in turn depends on the reactivity of the composed atoms and the stacking ways of the molecules under pressure.” Dr. Zheng Haiyan explained.“All the atoms on the benzene ring are carbon atoms, all actively participating in the polymerization reaction at high pressure and leading to many possible reactions. Therefore, we need to improve both the selectivity of aromatic ring reactions and their stacking order to obtain desired nanothreads.”
Compared with benzene, s-triazine has three nitrogen atoms alternately arranging with three carbon atoms on the ring. Because nitrogen atoms are not as active as carbon atoms under high pressure, it is difficult to form bonds between nitrogen and nitrogen, thus reducing the possible reaction pathways and promising to synthesize ordered crystalline nanothreads.
Therefore, the team used s-triazine as raw materials. They compressed s-triazine at ~ 10 times normal atmospheric pressure and 573 degrees in Kelvin, they obtained crystalline C3N3H3 CNTh with perfect hexagonal orientation and stacking. Using multiple characterizations combined with theoretical calculations, the team confirmed that the s-triazine underwent a new synergistic addition reaction by C-N bonding between three carbon atoms and three nitrogen atoms from neighbored molecules, occurring continuously along the stacking direction and finally crystallized to so-called tube (3,0) type CNThs. This is completely different to the classical reaction named Diels-Alder, normally happens in the aromatics under high pressure.
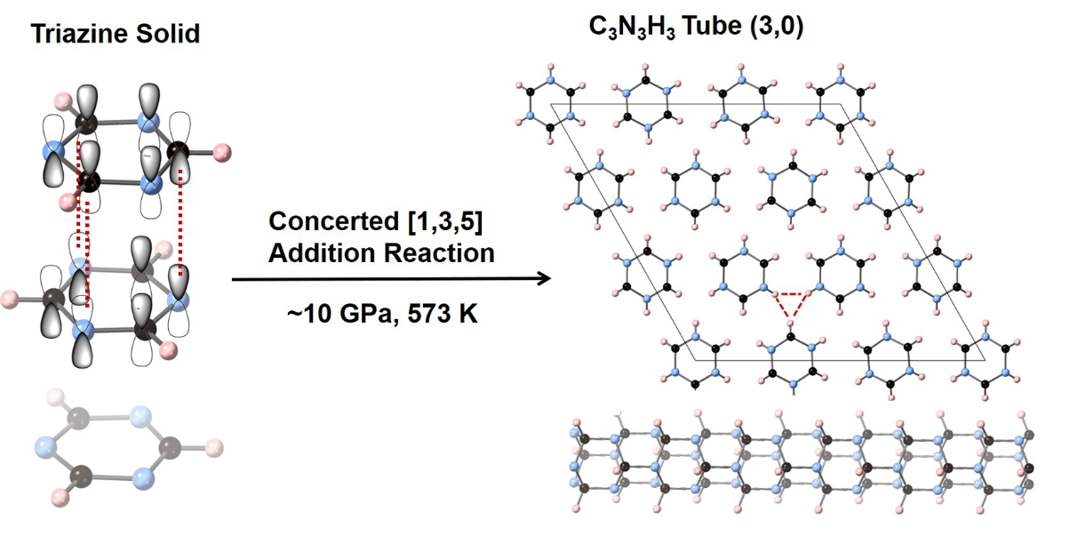
"The structure-specific crystalline CNTh with intrathread and interthread azimuthal ordering will realize extraordinary properties. The distinctive reaction selectivity and the regular molecular packing promote the reaction toward the ordered nanothread,” said Dexiang Gao, the co-lead author of the study.
Caption: Synthesis of crystalline C3N3H3 tube (3,0) nanothread through a high-pressure concerted [1,3,5] addition reaction between triazine.