
Valence change commonly happens in rare earth metals, while for Eu, it seems an exception in this group of metals. A team of scientists led by Dr. Yang Ding from HPSTAR published a study in the June 27 issue of the journal of Physical Review Letters confirming that a valence transition also occurred in Eu around 80 GPa along with a structural variation. Their study solved a long-standing debate regarding this question.
Valence transition ¾ the number of charge electrons variation, is a common phenomenon in rare earth elemental metals and compounds, which reflects the delocalization of the localized out-shell electrons, a remaining challenging topic in condensed matter physics. While Eu, a unique member in the rare-earth group, whether there is a similar valence transition like what does in the other rare-earth metals has been a long-standing puzzle under debate.
To solve this puzzle, the team of scientists applied state-of-the-art resonant x-ray emission spectroscopy and x-ray diffraction techniques to reveal the detailed electronic and crystal structural variations up to a record pressure of 160 GPa. They discovered a valence change in Eu around 80 GPa. And the valence transition just coincides with a crystal structural change in Eu around the same pressure.
“The resonant x-ray emission spectroscopy is so far the most powerful technique for studying valence states at high pressure, which give us the robust measurement of the electronic structure and thus detect the valence change in Eu,” said Dr. Ding.
Then what caused the valence change in Eu under compression?
“Pressure will reduce the distance between atoms and thus the distance between electrons in different orbitals and strengthen the overlapping between electrons. When the pressure is high enough, to around 80 GPa, one localized electron in 4f electron shell¾the third-out-shell of the Eu atom, within some Eu atoms will delocalize from the 4f orbital and jump to the neighboured, similar-energetic 5d band, which make the number of conduction electrons in Eu becomes a mixed between two and three,” explained Dr. Ding. “We can attribute the valence transition in Eu to the so-called promotional model ¾ 4f electron jumps in the 5d conduction band to induce a valence change .”
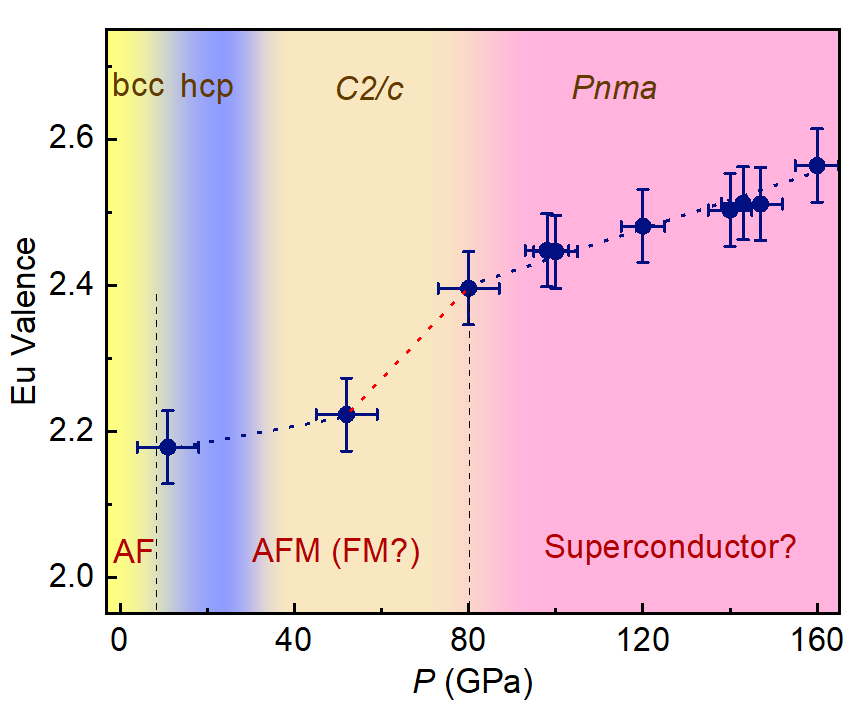
“When the pressure exceeds the critical value of valence transition, the valence-transition related phase transition strongly suppresses both the Hund coupling and Heisenberg coupling, giving rise to a metal-like system. Thus, the possible superconductivity in Eu metal is likely to originate from the valence instability around 80 GPa, and the low Tc value is likely due to the Eu metal not being fully trivalent.” added the lead author of the study, Dr. Bijuan Chen, a postdoctoral fellow at HPSTAR.
Therefore, their work provides important information of the physics underlying the unconventional superconductivity in the strongly correlated electron systems.
Caption: Pressure dependence of Eu valence at room temperature as determined by RXES.