
Title: Crystallographic Innovations in material science under extreme conditions
Time: 10:00 - 11:00 AM, Wednesday, November 18, 2015
Place: Auditorium Room 410, HPSTAR (Shanghai)
Host: Dr. Mao
Abstract:
Signification of crystallography
Crystal structure analyses experimentally determined the arrangement of atoms in the crystalline solids. Before the development of X-ray diffraction crystallography, the study of crystals was morphological measurements of their geometry. This involved measuring the angles of crystal faces and axes, and establishing the symmetry of the crystal. Crystallographic methods now depend on analysis of the diffraction patterns of a sample targeted by a beam of some type. X-rays are most commonly used; other beams used include electrons or neutrons.
Since synchrotron radiation with highly collimated beam and strong source intensity was facilitated, structure analyses are expanded not only for conventional atomic positional arrangements in the crystal but also electronic state and phonon information in the crystal. The following properties of the electromagnetic wave can be utilized for the analyses of the insight research of material sciences under extreme conditions. Interaction between electron and phonon under high pressure can be elucidated by combination with several experiments.
Electromagnetic wave characterExperiment
Diffraction …………………………….. powder and single crystal diffraction
Absorption …………………………….. EXAFS XANES IR Raman
Resonance ………………………….. Mössbauer XPS
Scattering …………………………….. XES
Only the diffraction technique of the wave can provide the detailed information of positional parameters (x y z) of the atoms and electrons in the 3 dimensional space. Structure analyses using X-ray, electron and neutron radiation give the different information by the interaction with the specimen in different ways.
• X-rays interact with the spatial distribution of electrons in the sample.
• Electrons are charged particles and interact with the total charge distribution of both the atomic nuclei and the electrons of the sample.
• Neutrons are scattered by the atomic nuclei through the strong nuclear forces between protons and neutrons, subatomic particles, and in addition, the magnetic moment of neutrons is non-zero. They are therefore also scattered by magnetic fields. When neutrons are scattered from hydrogen-containing materials.
Crystallography is useful in phase identification. When performing any process on a material, it may be desired to find out what compounds and what phases are present in the material. Each phase has a characteristic arrangement of atoms. X-ray or neutron diffraction can be used to identify which patterns are present in the material, and thus which compounds are present. Crystallography covers the enumeration of the symmetry patterns, which can be formed by atoms in a crystal and for this reason has a relation to group theory and geometry.
Crystallography in Material Sciences
Crystallography is one of the basic sciences and therefor it faces to many interdisciplinary sciences. In these days the application of crystallography is enormously expanded even in biological science. It accomplishes a large role for the researches of not only condensed matters but also amorphous materials even for structure researches of liquid and gas.
Physical properties of crystalline materials including amorphous materials under high pressure are reflected by (1) Elastic property and structure stability, (2) Lattice compression at high pressure, (3) Electron-phonon interactions
Crystallographic interests for material science are
ⅰ. Dynamical observation together with static structure.
ⅱ. Structure stability under extreme condition.
ⅲ. Structure transformations under nonambient conditions and their mechanisms.
The following diagram indicates the sciences strongly relayed to the crystallography:
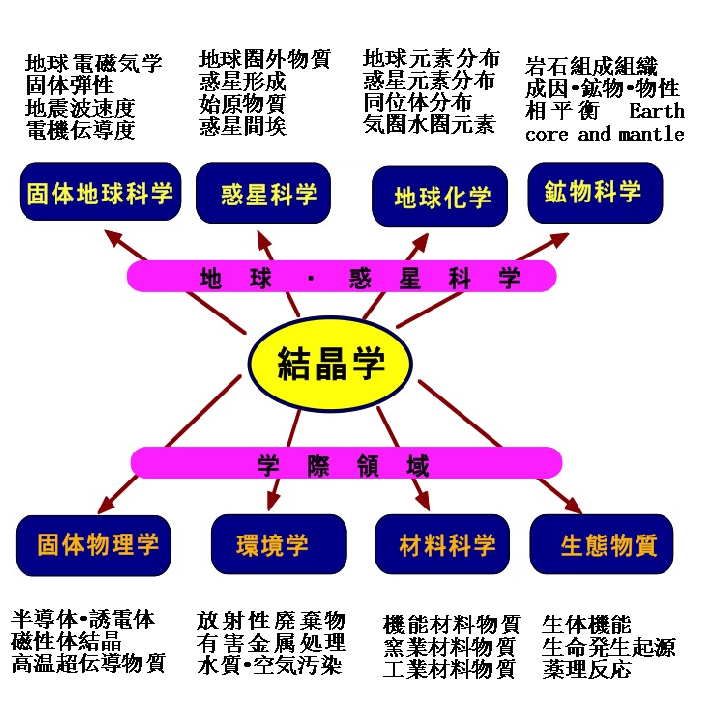
Microscopic to macroscopic structure in high-pressure sciences
Historically crystallographers made large efforts to analyze the structure mainly for atomic arrangement and metrical discussions. However, elements including electros and spins is not more than present in the substances and also physical field conditions. The electric, magnetic potentials and elastic force exist around these particles. These physical field conditions provide and control the bonding nature between atoms in nearest neighbor atoms, subsequently second, third neighbors and so on. Finally the atomic arrangement makes a long range ordered structure.
The following equation of Virial theorem can explain the high-pressure experiment.
Pext in the left term represents the external pressure, which we can control by experimental pressurization.
Fij•rij in the right term indicate potential of the bulk crystal.
This equation infers the high-pressure experiment by the equilibrium between the right and left term. Fij is an internal force between j and i particles. And rij is the interatomic distances. Summation of Fij・rij provides the total potential in the bulk crystal.
The equation explains the high-pressure experiments. High-pressure apparatus in the laboratory controls the pressure Pext to the substance in the sample chamber at the desired pressure. The pressure gives an effect to the potential in the chamber. Consequently the interatomic distances and bonding forces of every atom stay in the equilibrium state. The summation of rij provides the high-pressure structure and crystallographic symmetries.
MO calculation and Mulliken charge
We can elucidate the change in the electron density distribution as a function of pressure. Ab initio calculation of the electronic structure is performed using the quantum chemical calculation program package of Gaussian-09 (Frisch et al. 2009). The program is prepared for ab initio and semiempirical calculations on atoms and molecules. It is imperative using semiempirical methods. Structural models were based on the atomic coordinates experimentally determined. The results of the calculation are visioalized by program GaussianView, which is the advanced and powerful graphical interface available for Gaussian-09, in order to import or build the molecular structures, set up, monitor and control Gaussian-09 calculations. The calculated electron density to adjust its spatial extent appropriates for the particular molecular environment.
The electronic structure, band structure, density of state and electron density distribution have been examined with emphasis on the covalency effects and bonding nature of many compounds. Their dynamical properties and local spin density approximation can be discussed using the MO calculation. The previous calculated optical band gaps and lattice structure parameters are in disagreement with the relevant experimental results. A number of different approximations–correlation functional including hybrid exchange techniques have been developed. Many methods are compared with previous quantum mechanical calculations and available experimental data. Especially good agreement with the experimental data has been achieved for hybrid functional. First-principles HF and DFT calculations using the computer program package was performed.
Biography of the Speaker:
 Prof. Takamitsu Yamanaka's experimental works have been focusing to X-ray diffraction, absorption and resonance using synchrotron radiation for 20 years in PF-KEK and SPring-8 in Japan. One of his science interests is crystal physics facing to electron-lattice and spin-lattice correlation in earth’s interiors under extreme conditions. His visit to the Lab aims at the improvement of high-pressure crystallography in Geophysical Laboratory.
Prof. Takamitsu Yamanaka's experimental works have been focusing to X-ray diffraction, absorption and resonance using synchrotron radiation for 20 years in PF-KEK and SPring-8 in Japan. One of his science interests is crystal physics facing to electron-lattice and spin-lattice correlation in earth’s interiors under extreme conditions. His visit to the Lab aims at the improvement of high-pressure crystallography in Geophysical Laboratory.
Takamitsu was a Professor in the Department of Earth and Space Science and Professor at the Center for Quantum Science and Technology under Extreme Conditions in Osaka University. He is Emeritus Professor of Osaka University. He is a Member of National Science Council of Japan belonging to Japanese cabinet since 2003. He is President of International Mineralogical Association (IMA). He in an active member of the organizing committee of International Union of Crystallography (IUCr-2008 at Osaka).