
FEBRUARY 5, 2018
Hydrogen-rich compounds have attracted attention because of their potential application in hydrogen storage and for high-Tc superconductivity. High-pressure methods have proven very effective in the search for new materials with high hydrogen contents. A new study led by Dr Jack Binns, a postdoctoral researcher in the group of Dr Ross Howie, reports the discovery of a new high-H2 content compound with an unprecedented formula of HI(H2)13. A combination of X-ray diffraction, Raman spectroscopy, and molecular dynamics were used to determine the structure and explain the extraordinary stability of this compound with increasing pressure. This study is published in Physical Review B.
Previously the group of Dr Howie have shown that hydrogen iodide (HI) decomposes when compressed above 10 GPa. By laser heating the resulting mixture of I2 and H2 above 25 GPa they have now synthesized the molecular compound HI(H2)13. This compound adopts the AB13 type structure which has been observed in a wide range of systems, from metallic compounds to opal gems, but never before in a molecular configuration at high pressure. The icosahedral clusters of H2 molecules isolate the HI molecule, resulting in an extreme stability range, from 9 to at least 130 GPa. By compressing HI-H2 mixtures at 300 K, the authors observed the formation of another molecular compound, H2(HI)2, which exhibits stability similar to that of pure HI.
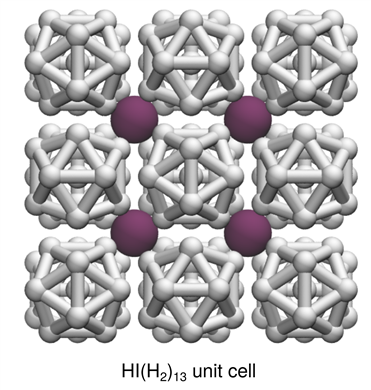
The structure of HI(H2)13 consists of a cubic lattice of HI molecules, within this lattice clusters of H2 molecules are arranged in well ordered clusters. By keeping HI molecules far apart, they are unable to decompose and this compound shows extraordinary stability. HI(H2)13 has an unprecedented 93 mol% H2 content is amongst the very highest achieved in molecular hydrogen storage materials.
“The unexpected formation of such a high hydrogen content compound raises an interesting question as to whether this structure could be stable with other molecules. If they are of the correct size it may be possible to stabilize the structure to ambient pressures, allowing us to store very large quantities of hydrogen” said Dr. Ross Howie.
This study involved Jack Binns, Philip Dalladay-Simpson, Mengnan Wang, and Eugene Gregoryanz of HPSTAR and was in collaboration with Graeme Ackland of CSEC, The University of Edinburgh.
Caption: HI(H2)13 crystal structure with the orientation of icosahedral clusters highlighted with “bonds” between H2 positions to guide the eye.
理论及实验指出富氢化合由于非氢元素的化学预压作用使得它们相对氢更容易实现金属化及超导转变。使得富氢化合物的研究成为了科学家们研究高温超导的热点课题。北京高压科学研究中心的Ross Howie研究员带领的小组通过高压的方法在富氢体系中首次合成了碘的富氢化合物HI(H2)13。该富氢化合物可以稳定保持到130 GPa。并且他们使用原位X射线衍射技术,拉曼光谱及分子动力学模拟确定了该化合物的结构。