
JANUARY 7, 2019
A research team led by Drs. Kuo Li and Haiyan Zheng from HPSTAR recently studied the pressure induced polymerization (PIP) of C6H6-C6F6 co-crystal, and an ordered substituted short range graphane, named as H,F-graphane was identified. They also investigated the reaction process and identified the Diels-Alder reaction is the elemental reaction for the synthesis. This provides an important reference for understanding and designing the reaction of aromatics under high pressure. The work is published on Angewandte Chemie International Edition (DOI: 10.1002/anie.201813120).
There are huge amounts of sp3carbon-based molecular species but quite few sp3 carbon materials. To synthesize more sp3 carbon-based materials, extreme pressure was applied on unsaturated molecules like aromatics. The C6H6-C6F6 (CHCF) cocrystal is a well-defined ordered molecular crystal due to the strong electrostatic attraction. By compressing this cocrystal, the authors found that it transformed into the H, F-graphane through the Diels-Alder reaction. This study provides an alternative method for constructing substituted graphane with atomic-level uniformity.
By using multiple neutron sources with constant wavelength and time of flight (TOF) under high pressure, the authors confirmed the crystal structure of CHCF at the reaction pressure threshold (20 GPa), which is the first example reported in aromatics. It shows the CHCF still keeps in stacked columns. The critical reaction distance between the aromatic molecules is around 2.8 Å.
"The crystal structure before the reaction is critical for us to understand the reaction process, and this is a great challenge for the organics due to their weak scattering to X-ray." said Dr. Kuo Li. "We use multiple neutron sources including ‘Fenghuang’ in China Mianyang Research Reactor, PEARL in ISIS, UK and PLANET in J-PARC, Japan to determine the structures."
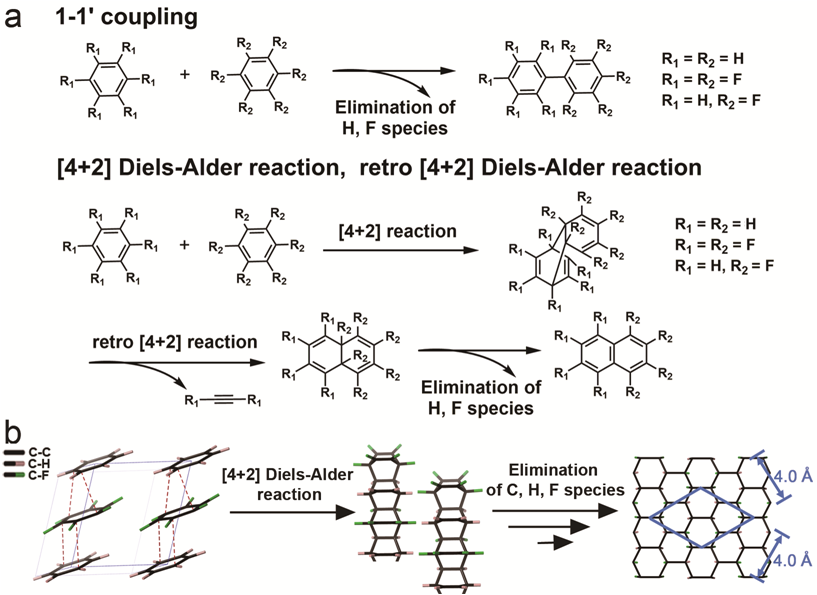 The authors also used IR, X-ray PDF, GC-MS, SEM, SAED, solid-state NMR and DFT calculation to investigate the product and the intermediates. The products found to be a short-range ordered hydrogenated-fluorinated graphane. [4+2] oligomers were detected by the GC-MS and hence Diels-Alder reactions were confirmed to be the elemental reaction leading to the graphane. It was proposed that the serial [4+2] Diels-Alder reactions happen between the alternately stacked C6H6 and C6F6 to form [4+2] polymers, and with the elimination of H, F species and connections between the neighbored polymers, the H,F graphane is generated.
The authors also used IR, X-ray PDF, GC-MS, SEM, SAED, solid-state NMR and DFT calculation to investigate the product and the intermediates. The products found to be a short-range ordered hydrogenated-fluorinated graphane. [4+2] oligomers were detected by the GC-MS and hence Diels-Alder reactions were confirmed to be the elemental reaction leading to the graphane. It was proposed that the serial [4+2] Diels-Alder reactions happen between the alternately stacked C6H6 and C6F6 to form [4+2] polymers, and with the elimination of H, F species and connections between the neighbored polymers, the H,F graphane is generated.
"The investigation on the high-pressure products and intermediates are extremely difficult due to their limited quantity, which actually hinders the development of high pressure synthesis." Dr. Haiyan Zheng said. "Here, we combined a variety of chemical tools to analyze the products, especially the GC-MS. It is very sensitive and can separate the mixture before detection, which provides a powerful method to detect the high-pressure products."
Caption: The reaction process and elemental reactions of PIP.
sp3杂化的碳骨架结构—尤其是具有石墨层状结构的饱和碳材料石墨烷及其修饰产物—因其优异的机械与光学性能而广受关注。近年来,高压极端条件(GPa级)提供了一种有效的聚合方法。北京高压科学研究中心的李阔、郑海燕课题组通过对苯-六氟苯1:1共晶进行压力诱导聚合反应,得到了短程有序的氟代石墨烷结构,并对反应机理进行了详细的研究。他们通过红外光谱、原位中子衍射、电镜、原子对分布函数以及气相-质谱联用等一系列表征手段并结合理论计算,对初始共晶结构、可能的中间产物及最后生成的石墨烷结构进行了严密的分析,并据此提出了以[4+2]Diels-Alder加成反应为主的反应机理。该研究为理解芳香类化合物的高压反应机理提供了重要实验证据,同时该结果还表明通过调控取代基可以得到多样的sp3杂化的碳骨架结构。该结果发表于近期的Angewandte Chemie International Edition (DOI: 10.1002/anie.201813120)上,第一作者为专业副研究员汪雅洁博士。