
APRIL 23, 2019
Scientists from HPSTAR and The University of Hong Kong combined in situ synchrotron x-ray diffraction and density functional theory and evolutionary algorithms to probe the possible new compounds in Pb-Te system. The team discovered a new cation-rich compound, Pb3Te2 in the binary system. This work is published in recent ACS Central Science.
Under ambient conditions, Pb atoms exhibit oxidation states of +2 or +4, and Te atoms often have a -2 valence state. So the binary compounds with 1:1 or 1:2 stoichiometires are expected to exist in the Pb-Te system. While at ambient conditions, we can only observe the 1:1 stoichimetric PbTe compound. So discovery of other new stoichimetry compounds in the Pb-Te system is a longstanding research topic.
We know that pressure could change the conventional stoichiometries and form some new forms of compounds that couldn’t under ambient conditions. For example, chemical inert helium could react with sodium at high pressures to form Na2He compound. In the Na-Cl system, sodium and chloride could form Na3Cl, NaCl3, and NaCl7 new compounds under compression. So what pressure will work on Pb-Te system is the focus of current research. The team of scientists is aiming to find other exotic compounds in the Pb-Te system which may have new potential practical applications.
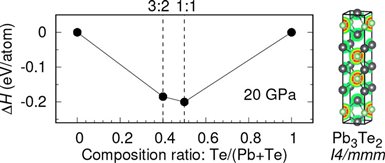
First, the team used theoretical calculation to find energetically stable compounds in the Pb-Te binary system up to 400 thousands atmosphere pressure and a new compound with an stoichiometry of 2:3 was predicted to be stable from 20 to 40 thousands atmosphere pressure.
“The large pressure range where Pb3Te2 is energetically preferable makes it might be possible to be synthesized,” said Dr. Kuo Li. So inspired by theoretical predications, the team of scientists observed the formation of the predicted new compound Pb3Te2 at some 20 thousands atmosphere pressures and ~1015 ℃ in a laser-heated diamond anvil cell from in-situ synchrotron x-ray diffraction. Unlike Pb-Te, Pb3Te2 is a cation-rich compound.
Their further electronic structrual calculation reveals that Pb3Te2 is metallic and will change to a superconductor at low temperature under compression. “Actually, this work is a continue of our previous discovery of Sn3Se4 in the group IV-VI,” said Dr. Kuo Li. “The successful synthesis of the new compounds paves the way for the discovery of other IV-VI compounds with non-conventional stoichiometries and novel properties.”
Caption: Enthalpies of formation of the predicted stable compounds in the Pb−Te binary system at 20 GPa and electron localization function of Pb3Te2 at 20 GPa.
PbTe是IV-VI主族半导体化合物中的典型代表。因其在光电,热电,存储转换方面的优异性能而受到人们的广泛关注。常压下,Pb-Te体系中只有PbTe一种化合物。那么,在该二元体系中是否存在与Na-Cl体系类似的、其它化学配比的新型化合物呢?近几年来的理论研究和实验表明,高压可以改变元素的化学配比,从而形成经典化学中不可能的化合物。北京高压科学研究中心的李阔研究员与香港大学陈粤教授小组合作,预测并合成了四六主族新型化合物Pb3Te2,该化合物具有金属特性,并且在低温下会转变为超导体。此新化合物的合成意味着第四-第六主族可能还有其他潜在的新型化合物以待发现。相关研究结果发表于ACS Central Science。