
A research team led by Dr. Hengzhong Zhang from the Center for High Pressure Science and Technology Advanced Research (HPSTAR) illustrated that the rate-determining step (RDS) of oxygen evolution reaction (OER) could be regulated by simply varying the cation and anion complexity in a family of the metal phosphorous trichalcogenide (MPTC) electrocatalysts (MPT3, where M = Fe, Ni; T = S, Se). Their study unveils the pivotal role of the cation-anion interactions in determining the catalyst performance and provides a simple way for predicting catalytic activities. Their findings were recently published in Angewandte Chemie International Edition (DOI: 10.1002/anie.202214570).
Oxygen evolution reaction is an important half-cell (anodic) reaction in water splitting. Generally, a large overpotential is required to drive the OER due to its sluggish kinetics, while the overall OER kinetics is known to be limited by a RDS among several elementary reactions in the OER mechanism. Thus, gaining the ability to control the OER RDS purposely would facilitate realizing high OER kinetics.
"Pure density-functional theory (DFT)-calculated free energy changes and/or experimental Tafel slopes were used commonly to infer the OER RDS. However, these methods suffer from large uncertainties due to the lack of transition states in the former and the non-uniqueness of the latter,” said Dr. Zhang. "We are still in dire need of dependable methods to determine and then intentionally control the OER RDS to speed up the OER kinetics.”
Using metal phosphorous trichalcogenides as the electrocatalysts for OER, the team led by Dr. Zhang set out to study the OER kinetics and then use microkinetic modeling to determine the OER RDS conclusively. They found that the OER kinetics increases in a certain order in their synthesized MPTC catalysts #1 – 6 (see diagram below), arising from a sequential increase in the kinetic rate constant of the RDS in the formation of the M-O* surface species on #1 – 5, and a change of the RDS to the formation of the M-OOH* species on the (Ni,Fe)P(S,Se)3 catalyst (#6).
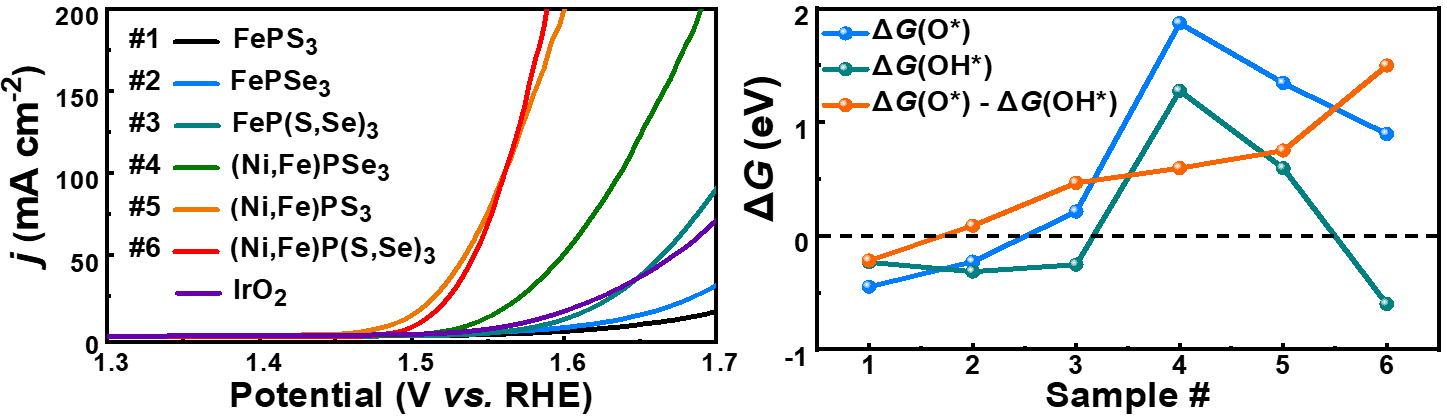
Caption: Geometric area-normalized polarization curves (left) and adsorption free energies and OER activity descriptor (right) of the synthesized metal phosphorous trichalcogenide electrocatalysts.
The team also carried out DFT calculations to understand the activity trend of these MPTC electrocatalysts better. Their calculated OER activity descriptor ΔG(O*) – ΔG(OH*) predicts an OER catalytic activity sequence of #6 > #5 > … > #1, in good agreement with the experimental results. In particular, (Ni,Fe)P(S,Se)3 (#6) has a nearly optimal OER activity descriptor value of DG(O*) – DG(OH*) = 1.5 eV, explaining its exceptionally high OER activity.
In addition, the team also found that the OER activity descriptor could be related to the chemical composition of the MPTC catalysts via a newly proposed anion/cation complexity index. The complexity index is the sum of the metal-S/Se bond contributions, and is proportional to the OER activity descriptor. This discovery makes it possible to predict catalytic activities simply from catalyst chemical compositions rather than expensive DFT calculations.
"Our work demonstrates a simple way to control the RDS and to greatly increase the OER kinetics by simply varying the cation/anion complexity in an electrocatalyst,” said Dr. Zhang. “Our findings will significantly expedite future catalyst discoveries.”
近日,北京高压科学研究中心的张衡中研究员团队的最新研究表明,析氧反应的速率和决速步骤可以简单地通过改变金属磷硫族化合物电催化剂(MPT3,其中M = Fe, Ni; T = S, Se)的阴阳离子复杂度来调控。与许多相关的析氧反应电催化剂相比,他们发现复杂度最高的化合物 — (Ni,Fe)P(S,Se)3呈现出超低的Tafel斜率和过电位,理论计算也表明,该催化剂具有近于最优的析氧反应活性描述符值。另外,他们发现不同催化剂的活性描述符值与阴阳离子复杂度指数 — 各阴阳离子键对贡献值的总和,表现出很好的线性关系。该研究揭示了阴阳离子相互作用对催化剂活性起着关键作用,从而为预测催化剂活性提供了一种简便快捷的方法。相关研究成果以“Expediting Oxygen Evolution by Optimizing Cation and Anion Complexity in Electrocatalysts Based on Metal Phosphorous Trichalcogenides”为题发表在《德国应用化学》上。