
Topochemical reaction is a powerful and promising method to obtain well-ordered polymeric materials with desirable optoelectronicand mechanical properties, in which the regio- or stereoselectivity is highly depended on the intermolecular distances at threshold. A recent work from a team led by Dr. Kuo Li from HPSTAR and Dr. Xiao Dong from Nankai University revealed the distance-vibration-based reaction mechanism and improved the understanding of reactions in solids. This work is published in the journal of Matter and Radiation at Extremes (Matter Radiat. Extremes 8, 058402 (2023); doi: 10.1063/5.0151609).
The intermolecular C…C distance (dC…C) was reported as a key parameter for pressure-induced polymerization (PIP) in organic molecular systems. The shortest dC…C at threshold (d(C…C)min) was ~3 Å at room temperature (RT), varing slightly with the functional groups involved in reactions. The team led by Drs. Kuo and Dong took the acetylene system as an example, using computational approaches to investigate the structural geometry changes, vibrational modes as well as reaction profiles under pressure, and successfully unraveled the “3 Å-mystery”.
The team first found an intrinsic threshold distance of 2.3 Å as the onset of intermolecular bonding of carbon atoms, and their subsequent investigation revealed how phonon modes decrease the dC…C and trigger the reaction at RT. The researchers illustrated the vibrational modes in the system by calculated phonon spectra and gave out a method to quantitate the thermal contribution of each mode. The total thermal contribution for one carbon atom could reach 0.4 Å, pushing the instantaneous minimum dC…C to 2.3 Å and pass the threshold. That also provided the enthalpy to overcome the energy barrier, thereby induced the reaction. Distance, vibration, and enthalpy were quantitatively correlated together in the interpretation, and the nature of threshold distance became clearer.
This is the first time that the underlying mechanism of topochemical reaction was demonstrated from the viewpoint of solid physics. This would bring insight into the thermodynamics and dynamics of PIP and provide a theoretical basis for controllable precision synthesis via topochemical reactions.
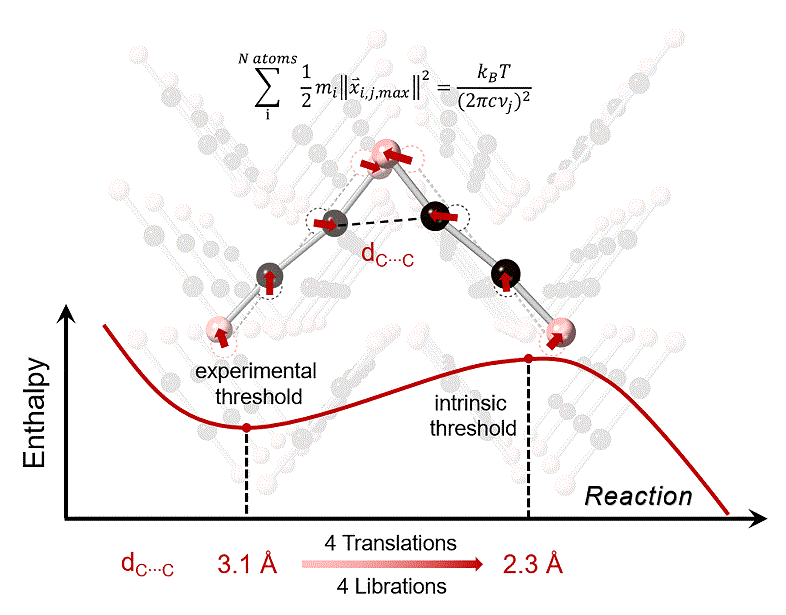
Caption: Vibration-triggered reaction in acetylene system.
拓扑化学反应是一种新型、有效的合成手段。分子晶体中反应前的最短分子间距(即临界距离)是反应的最重要特征之一,直接决定了拓扑化学反应的立体选择性。对临界距离本质的研究能加深我们对固体化学反应的理解,并帮助我们更好地设计、合成功能性材料。近日,北京高压科学研究中心的李阔、郑海燕课题组与南开大学的董校课题组通过计算理论研究了乙炔的高压拓扑聚合反应,首次深入探究了反应的动力学引发过程,定量化给出了热振动的贡献,解释了实验规律并给出了临界距离的本质。相关结果近期发表于《极端条件下的物质与辐射》(doi: 10.1063/5.0151609),文章第一作者为北京高压科学研究中心博士生汤星宇。