
MAY 13, 2020
If we took a journey from Earth’s surface to the center, the midway point locates roughly at 1900 km depth in the lower mantle. The lower mantle ranges from 660 to 2900 km depth and occupies 55% of our planet by volume. The chemical composition of the lower mantle is rather simple. It has long been pictured as being made up of 2 major minerals (~95%), namely bridgmanite and ferropericlase. Until recently, this neat model is directly challenged by a set of discoveries in the lower mantle.
 |
“One of the major lower mantle compositions, ferropericlase (Mg,Fe)O, turns into a pyrite-type structure upon meeting water. This intriguing chemical reaction only occurs at Earth’s deep lower mantle which is defined in depths between 1900 and 2900 km” said Qingyang Hu from HPSTAR. “The reaction produces so-called oxygen excessive phases, or simply superoxides. The lower mantle is oxidized in the presence of water.” Generally, when all the oxygen atoms in a compound are bonded with metal atoms, they are called oxides. However, if a compound has paired oxygen atoms, like oxygen-oxygen bonding, it becomes a superoxide. Although superoxide is rarely found in nature, it might be common in Earth’s deep lower mantle.
“We also found that olivine and its high-pressure phase wadsleyite, the dominating minerals in the upper mantle, decompose to generate superoxides when subducting down into the deep mantle with water.” added by Jin Liu from at HPSTAR. Few approaches are available for scientists to probe into the lower mantle mineralogy given its depth. “Our experiments are very challenging. We input appropriate parameters like pressure, temperature, and starting minerals. Then we investigated the outputs including chemical reactions, new mineral assemblages, and their density profiles. Those parameters allow us to better constrain the nature of the lower mantle and its oxidation state.” Contrary to the paradigm that the lower mantle is highly reduced, our results indicate that the deep lower mantle is at least locally oxidized wherever water is present.
The team members proceeded with minerals existing on Earth’s surface, by squeezing them between two pieces of diamond anvils to generate about 100,000,000 times the atmospheric pressure at sea level, heating them up using infrared laser, before analyzing the samples using a battery of x-ray and electron probes. The experiments have mimicked the extreme pressure-temperatures conditions found in Earth’s deep lower mantle.
Previous experiments explored a dry mineral assembly in the absence of water. Those experiments reported that bridgmanite (and/or post-bridgmanite) and ferropericlase are the most abundant and stable minerals throughout the lower mantle. However, when water is introduced, ferropericlase would be partially oxidized to superoxide under the deep lower mantle conditions. The superoxide is verified to stay in harmony with bridgmanite and post-bridgmanite.
This new water-mantle chemistry can be closely linked to the water cycling in the solid Earth. Every year, billions of tons of ocean water falls into the deep Earth at tectonic plate boundaries. While some water returns via underwater volcanoes and hot vents, others goes deep into the Earth’s interiors. “Our experiments indicate the deep water is an essential part of mantle chemistry. The water cycling can extend to the deep lower mantle where water has extraordinary oxidation power, producing highly oxidized superoxide and releasing hydrogen.” suggested by Dr. Ho-kwang Mao from HPSTAR. “The lower mantle can be oxidized and reduced at the same time.”
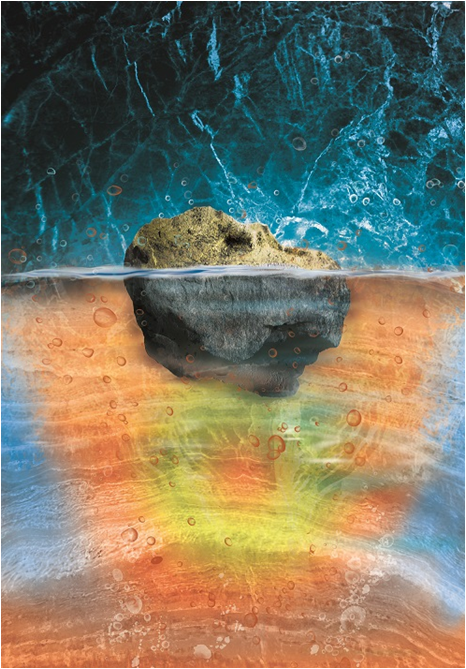
Caption: The schematic artwork shows a boundary within the lower mantle at the depth of 1900 km. Below 1900 km, the interaction between water and mantle is triggered.
The study is published on National Science Review.
Media Report:
EurekAlert: https://www.eurekalert.org/pub_releases/2020-05/scp-tlm052220.php
Phys.Org: https://phys.org/news/2020-05-earth-mantle-oxidized-presence.html
下地幔占地球体积的55%以上,与地壳和上地幔复杂的矿物组成不同。当前研究认为下地幔95%以上由布里奇曼石和铁方镁石组成。北京高压科学中心毛河光院士团队自2016年起,提出了铁、氧两大元素在1700 km深度以下可以形成稳定的高压黄铁矿相FeO2。在高温高压条件下通过与水(或含水物质)反应,铁单质与铁氧化物均被氧化形成稳定的含氢FeO2,这一新相的出现在一定程度上丰富了下地幔的矿物组成。近期,这一团队的胡清扬和刘锦研究员通过激光加热结合同步辐射X光衍射技术,发现除了铁氧化物外,下地幔主要成分之一的铁方镁石也可以与水发生化学反应。在下地幔深部,即在距离地表1700-2900 km深度的温度和压力条件下,铁方镁石与水反应生成(Mg,Fe)O2。该矿物相与新近发现的(Mg,Fe)2O3+x一起均可归类于富氧氧化物。在地球表面,这类富氧氧化物的化学性质通常不稳定,而在高压环境下,富氧氧化物稳定性显著提升,可以说地球深部的高温高压环境提供了富氧氧化物稳定存在的物质条件。该研究小组还发现富氧氧化物与地球内部水的循环紧密相关,当水或含水矿物到达了一定深度,如1700 km以下,与地球深部的主要矿物发生氧化还原反应,在生成(Mg,Fe)O2的同时释放氢气,而氢气在上升的过程中又会重新与氧元素结合,重组为水。水在这一往复循环中总量大致不变,起了类似催化剂的作用。水不但是生命活动必需品,也在地球内部的化学平衡起关键作用,是地球宜居的最重要原因之一。