
NEWs and Research Highlights
 | Complex Phase Behaviour of Dense Chlorine- Philip Dalladay-Simpson & Ross Howie MARCH 14, 2019— Due to the realization of potentially exotic states, the behaviour of elemental molecular systems at high densities has long been of particular interest in the condensed matter sciences. Of the 7 elemental diatomic systems, only O2 and the heavier halogens Br2 and I2 have conclusively demonstrated electrically conductive properties in the condensed state, whilst the latter two are the only examples of diatomic molecular dissociation. In a new investigation, an international collaboration between scientists from HPSTAR, the University of Edinburgh and GeoSoilEnviroCARS (GSECARS, APS), report the behaviour of chlorine to pressures in excess of several million atmospheres. The study, which is published in Nature communications, reveals the peculiarity associated high-pressure chlorine, such as metallic modification, structural complexity and ultimately molecular dissociation.[Link] | ||||||
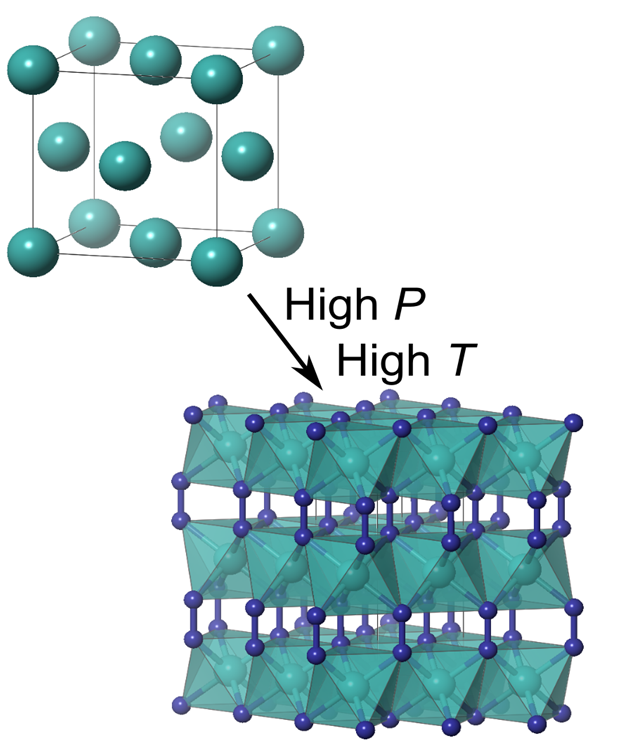 | Direct Reaction between Copper and Nitrogen at HP and HT– Jack Binns MARCH 6, 2019 — Compounds of the transition metals and nitrogen have applications in many areas from ultrahard materials to explosives. To date none of the group 11 elements: copper, silver, or gold are known to form a nitride compound, MN2 despite many decades of attempts. A new study by RTH Lab of HPSTAR reports that by laser heating mixtures of the elements in diamond-anvil cells at high pressures, a direct reaction between copper and nitrogen is observed forming the new compound copper diazenide (CuN2). This study is published in The Journal of Physical Chemistry Letters.[Link] | ||||||
 | 98% of the Universe as Unreactive as Ever - Dr. Ross Howie NOVEMBER 13, 2018— Extreme conditions are very common in the universe, from the cold vacuum of deep space to the elevated temperatures and pressures within planets. The gas-giant planets, Jupiter and Saturn, consist primarily of hydrogen and helium, whilst the nitrogen-hydrogen bonded compound ammonia, is abundant on Saturns icy moons, Titan and Enceladus. This study is published in Physical Review Letters.[Link] | ||||||
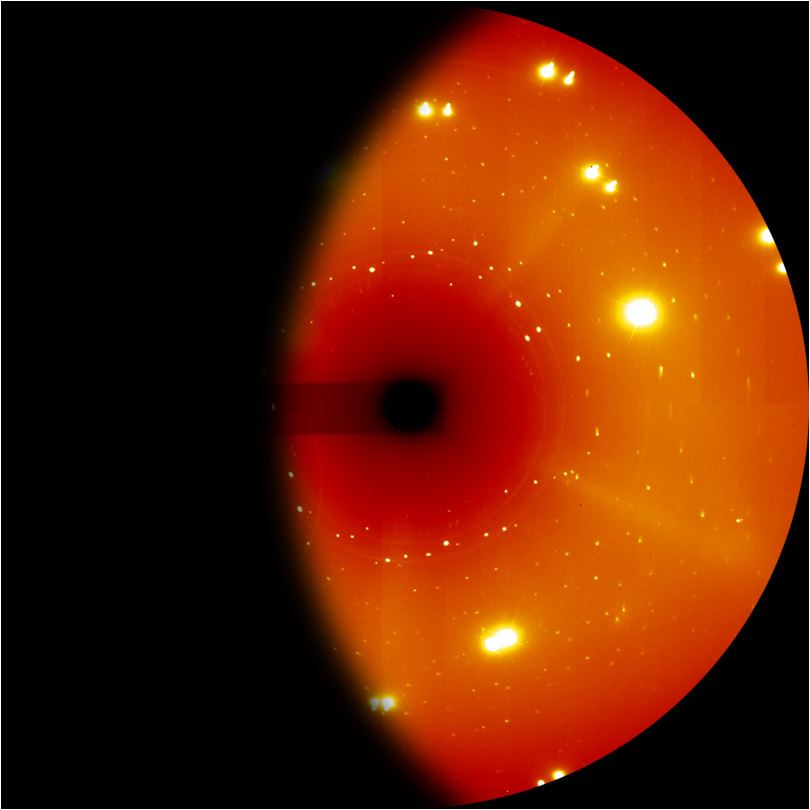 | Elusive Nitrogen Phase Exhibits Unexpected Complexity – Drs. Ross Howie & Eugene Gregoryanz NOVEMBER 12, 2018 — Although one might think that nitrogen, the primary constituent of the air which we breathe, is simple, it exhibits rich polymorphism across a wide range of extreme pressures and temperatures. Here a research team including HPSTAR members Jack Binns, Ross Howie and Eugene Gregoryanz, were able to experimentally resolve one of the most elusive forms of this model system, the dense solid iota-phase, that can only be accessed through a high temperature synthesis route. This study is published in Nature Communications.[Link] | ||||||
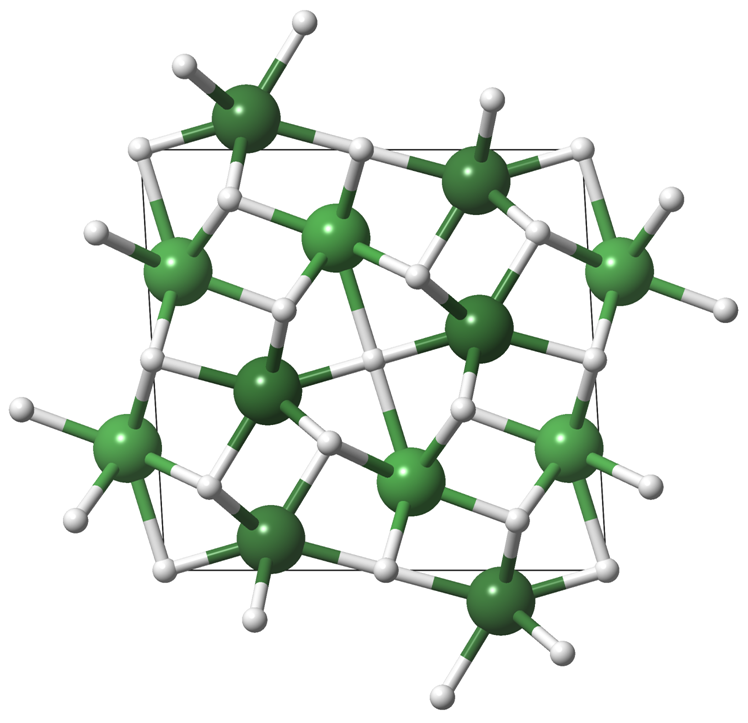 | The first nickel polyhydride Ni2H3 - Dr. Jack Binns OCTOBER 11, 2018— Combining high pressures and temperatures can lead to the formation of unusual new compounds. A new study by a group of HPSTAR scientists applies this method to the search for new transition metal hydrides, finding the first nickel polyhydride Ni2H3. This study was published as a Rapid Communication in Physical Review B.[Link] | ||||||
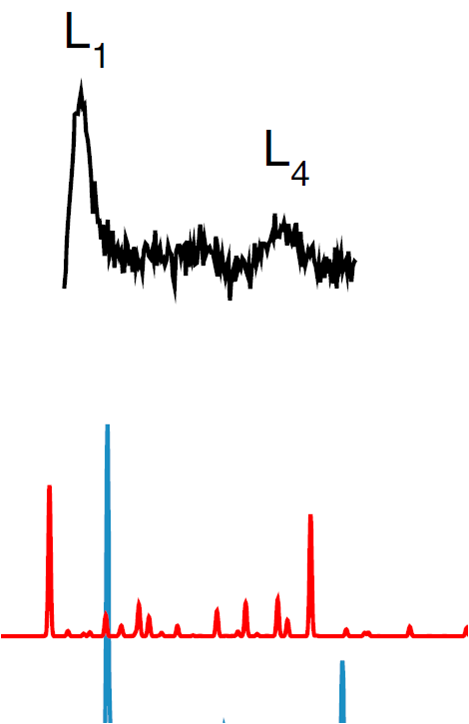 | Structure and Metallicity of Phase V of Hydrogen - Drs. Philip Dalladay-Simpson, Ross T. Howie, and Eugene Gregoryanz JUNE 18, 2018 — A new study from a team of scientists, which comprised of HPSTAR’s Philip Dalladay-Simpson, Ross T. Howie, and Eugene Gregoryanz, proposed a promising candidate crystal structure for their recently discovered phase of hydrogen, phase V. Most poignantly the work, which was recently published Physical Review Letters, provides further evidence that phase V is indeed a stepping stone towards hydrogen’s elusive metallic form. This study is published in the Physical Review Letters.[Link] | ||||||
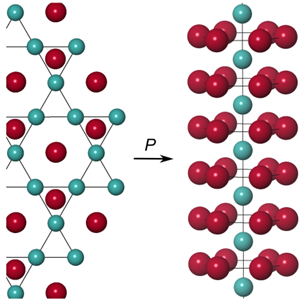 | Enhanced reactivity of lithium and copper at high pressure - Dr. Jack Binns JUNE 6, 2018 — High pressure can profoundly affect electronic structure andreactivity, creating compounds between elements that do not react at ambientconditions. Lithium is known to react with gold and silver, however no coppercompounds are known to date. New study by a group of HPSTAR scientists reportsthat by compressing mixtures of the elements in diamond-anvil cells, compoundsof lithium and copper have been synthesized for the first time. This study is published in the Journalof Physical Chemistry Letters.[Link] | ||||||
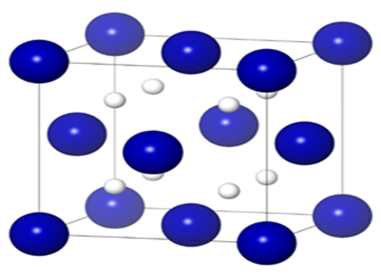 | High pressure synthesis and stability of cobalt hydrides - Mengnan Wang APRIL 18, 2018 — The synthesis and properties of hydrogen-bearing compounds is an intensively studied area of physics and chemistry, mainly driven by hydrogen-storage applications. High pressure and high temperature have been an effective route to synthesis hydrides with extremely high hydrogen content. A new study by RTH lab, reports the discovery of new cobalt compounds, CoH and CoH2, with high hydrogen content of 3.3 wt% and a volumetric hydrogen density of 214g/L. This study is published in J. Chem. Phys.[Link] | ||||||
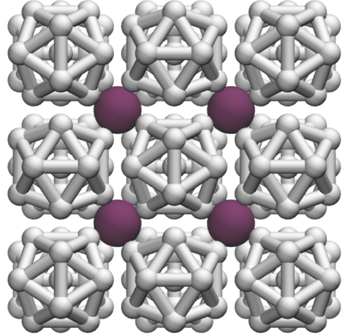 | Formation of H2-rich iodine-hydrogen compounds at high pressure - Dr. Ross Howie FEBRUARY 5, 2018 — Hydrogen-rich compounds have attracted attention because of their potential application in hydrogen storage and for high-Tc superconductivity. High-pressure methods have proven very effective in the search for new materials with high hydrogen contents. A new study led by Dr Jack Binns, a postdoctoral researcher in the group of Dr Ross Howie, reports the discovery of a new high-H2 content compound with an unprecedented formula of HI(H2)13. A combination of X-ray diffraction, Raman spectroscopy, and molecular dynamics were used to determine the structure and explain the extraordinary stability of this compound with increasing pressure. This study is published in Physical Review B.[Link] | ||||||
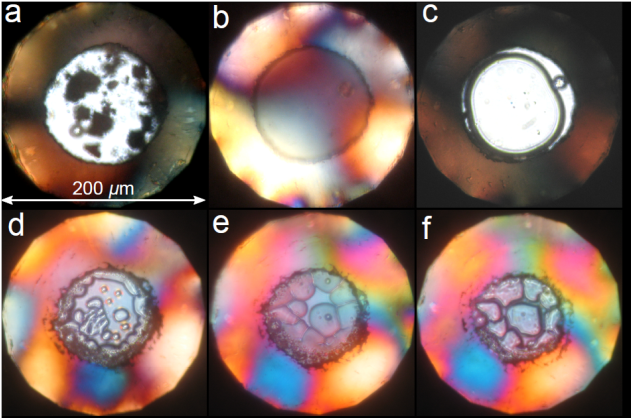 | Synthesis and stability of hydrogen selenide compounds at high pressure - Dr. Ross Howie NOVEMBER 20, 2017 — The observation of high-temperature superconductivity in hydride sulfide (H2S) at high pressures has generated considerable interest in compressed hydrogen-rich compounds. The heavier hydrogen chalcogenides (i.e., H2Se and H2Te) are predicted to also exhibit high Tc superconductivity, however up until now remained experimentally unexplored. A new study led by Dr. Ross Howie of HPSTAR investigated the synthesis and stability of H2Se at high pressure and finds remarkable similarities with H2S. This study is published in J. Chem. Phys.[Link] | ||||||
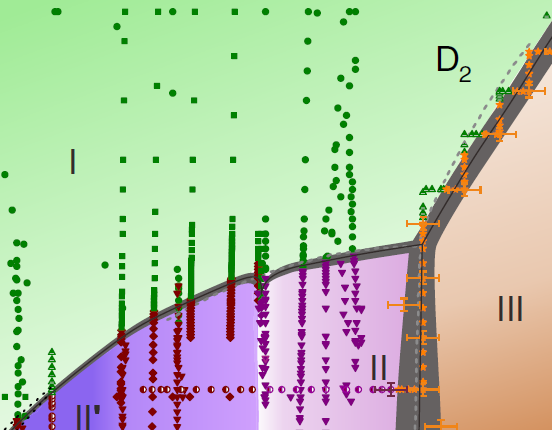 | High-Pressure Behavior of Hydrogen and Deuterium at Low Temperatures - Drs. Ross Howie, Xiaojia Chen and Eugene Gregoryanz AUGUST 17, 2017— New work by HPSTAR’s Ross Howie, Xiao-Jia Chen and Eugene Gregoryanz, in collaboration with the ISSP Chinese Academy of Sciences, presents the first high-pressure experimental study of hydrogen that was completely conducted within China. The authors present in situ high-pressure low-temperature high-quality Raman data for hydrogen and deuterium where they demonstrate the presence of a novel phase, phase II’, unique to deuterium. This work is published Physical Review Letters (DOI: 10.1103/PhysRevLett.119.065301).[Link] | ||||||
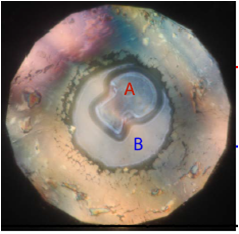 | Formation of Xenon-Nitrogen Compounds at High Pressure - Dr. Ross Howie OCTOBER 17, 2016 – A group led by HPSTAR scientist Dr Ross Howie and including Dr Jack Binns (RTH Lab) and Dr Phillip Dalladay-Simpson (CEP), in collaboration with researchers from CSEC (Edinburgh, UK) used high pressures to explore the possibility of forcing two of the more unreactive elements of the periodic table, xenon and nitrogen, to react. Joint investigation by X-ray diffraction and Raman spectroscopy showed the formation of a novel van der Waals compound at pressures as low as 5 GPa. After transformation to a lower symmetry phase this material, Xe(N2)2, remains remarkably stable up to at least 180 GPa and temperatures of 2000 K. This study is published in the journal Scientific Reports (doi:10.1038/srep34896).[Link] | ||||||
 | Glimpse of metallic hydrogen - Dr. Ross Howie JANUARY 7, 2016 — Driven by the predication of metallic hydrogen, the first and simplest element, there has been 80 years’ worth of combined theoretical and experimental effort to try to reach this predicted state in hydrogen. New breakthrough work from a team including Philip Dalladay-Simpson, and Ross T. Howie, two new scientists of HPSTAR, have carried out static compression on hydrogen and its isotopes above 380 gigapascals; higher pressure than any previous study. The work indicates another new phase, phase V, is detected in both hydrogen and hydrogen deuteride at pressures above 325 gigapascals at room temperature. Phase V could provide a glimpse of the theoretical predicated metallic hydrogen. This breakthrough discovery is published on January 07, 2016, in Nature.[Link] | ||||||
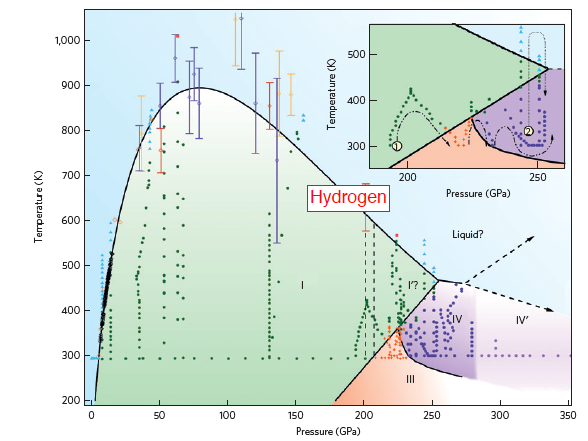 | Elusive Hot Hydrogen Leads To More Pressing Questions - Dr. Ross Howie MARCH 3, 2015 — Scientific studies of hydrogen at extreme pressures and temperatures are crucial for understanding the rich variety of unusual states and structures, and strange properties, that dense hydrogen demonstrates. But the combination of high pressures with moderate temperatures (>200 GPa and 550 – 1,300 K) has long been an experimentally inaccessible "no man's land" for hydrogen. Publishing in Nature Materials (DOI: 10.1038/NMAT4213), Dr. Ross Howie, a new Associate Staff Scientist who is joining HPSTAR in 2015, and co-workers at the University of Edinburgh have demonstrated now to overcome this difficulty (>200 GPa and 550 – 1,300 K), allowing them to observe a new, dense form of hydrogen, possibly a liquid phase at unusually low temperature. | ||||||
Last update April 26,2019